α2 nor-adrenergic receptor agonist
α2 nor-adrenergic receptor agonistEmploying a novel mechanism of action, RE03 is the only insomnia drug candidate in clinical development to target noradrenergic signaling, a neurological process which causes elevated arousal and wakefulness under stress.
Based on healthy human study results, RE03 has demonstrated an increase and consolidation in NREM and REM sleep, leading to an overall improvement in sleep architecture, restorative sleep and daytime functioning, the hallmarks of natural regenerative sleep. Study results indicate these benefits are achieved without the hangover effects or orthostatic dysregulation typical of most sleep medications. With its low side-effect, tolerance and dependency risk profile, and its ability to improve comorbid psychiatric symptoms, RE03 offers the potential of a disease modifying, first-in-class treatment for insomnia in PTSD, and stress-induced primary insomnia.
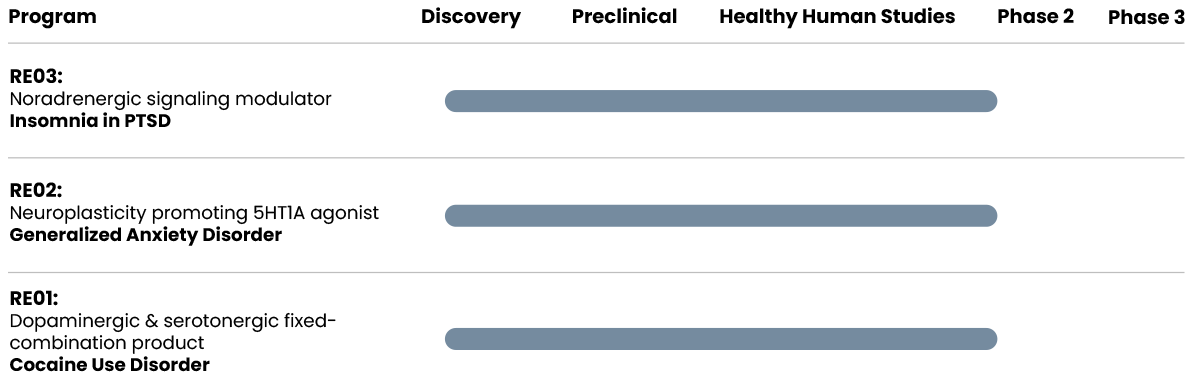
Development status – next steps: Phase 2a clinical trial in insomnia in PTSD.
 Neuroplasticity promoting 5-HT1A agonist
Neuroplasticity promoting 5-HT1A agonistRE02 has been engineered to entirely remove the psychedelic effects of the compound and to maximize the therapeutic effects of 5-HT1A agonists within an accessible, well-tolerated, and scalable sublingual administration.
Based on healthy human study results, RE02 has demonstrated highly improved pharmacokinetic profile and highly differentiated pharmacodynamics as compared to other compounds in this the same class of molecules.
Development status – next steps: Phase 2a clinical trial in treatment-resistant generalized anxiety disorder (GAD).
 5-HT2A agonist + dopamine modulator, fixed-combination
5-HT2A agonist + dopamine modulator, fixed-combination Based on healthy human study results, RE01 has demonstrated superior bioavailability, and an improved pharmacokinetic and pharmacodynamic profile in comparison to other 5-HT2A agonists in the same class of compounds.
Administered via Reconnect’s exclusively licensed sublingual technology in combination with a precision dosing regime, RE01 provides a highly controllable and precise therapeutic dosing range targeting select neurobiological and psychological mechanisms of action. Formulated to minimize unpredictable and adverse changes to perceptual and cognitive functions, RE01 offers the potential to provide immediate symptom alleviation and long-term disease modification with few side effects.
Development status – next steps: Phase 2a clinical trial in cocaine use disorder.